Background: Chimeric antigen receptor (CAR) T cell therapy has achieved remarkable efficacy in children and young adult (CAYA) B-cell acute lymphoblastic leukemia (B-ALL). Nonetheless, this therapy is highly personalized, technically complex, and costly to manufacture. The USA FDA approved the first CAR T cell therapy (Kymriah) in Aug 2017, for relapsed/refractory CAYA B-ALL. The list price of Kymriah is $475,000, not accounting for costs related to delivery of care, inpatient hospitalization, toxicity management or follow-up. A half-decade of clinical experience has amassed since commercialization, yet financial analyses remain sparse. Here, we conduct the first study to understand costs for commercial CAR in CAYA B-ALL.
Methods: Ten centers participating in both the Pediatric Real-world CAR Consortium (PRWCC) and Pediatric Health Information System (PHIS; Children's Hospital Association, Lenexa, KS) were eligible for study (08/2017-03/2020). Clinical data from the PRWCC database was merged with PHIS using age, diagnosis, center and lymphodepletion dates. Utilizing center and year specific charge-to-cost ratio, PHIS provided data for inpatient admission associated costs, but not outpatient care. For patients that were infused outpatient and subsequently admitted, only inpatient cost was available and analyzed for the first 60 days post-infusion. For patients that were infused inpatient, cost was calculated from the first day of admission for lymphodepletion or Kymriah infusion to 60 days post-infusion. The 60-day follow up was chosen as most of the clinical activity and toxicities occur within the 60 days post-infusion. Each patient was assigned a cost of $475,000 for Kymriah (list price). For the subset of patients who underwent allogeneic hematopoietic cell transplantation (alloHCT) following Kymriah, the median inpatient cost was assessed for 1-year post-alloHCT. Cost data was analyzed as medians and ranges. Univariable and multivariable logistic regression models were used to identify factors associated with the higher median cost associated with inpatient admissions.
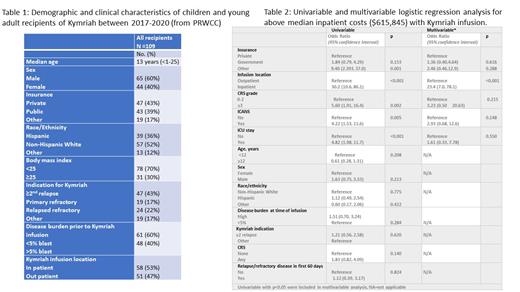
Results: 109 Kymriah-recipients were included. Patient characteristics are listed in Table 1. The incidence of grade 1-2 and ≥3 CRS was 42% and 23%, respectively, and grade 1-2 and ≥3 ICANS was 17% and 6%, respectively. Relapse/refractory disease at day 30 and 60 post-Kymriah infusion was 11% and 16%, respectively. AlloHCT was performed in 17 (16%) patients. Median time to alloHCT from Kymriah infusion was 148 days (36-354).
The median number of admissions was 1 (0-5) and median length of all hospitalization was 18 days (0-69), 99% patients required inpatient admission. Thirty-six patients (33%) required intensive care unit (ICU) admission with a median ICU stay of 5 days (1-24). The median total cost (Kymriah + inpatient cost) per patient was $615,845 ($475,000-1,578,321). Breakdown of median total cost (excluding Kymriah) was: room/board $33,676 ($0-454,256), pharmacy $65,854 ($0-515,132), laboratory/pathology cost $10,496 ($0-201,900) and other cost $8,204 ($0-573,163). One-year median cost associated with inpatient admissions for alloHCT was $556,712 ($105,670-1,919,275), combined median cost associated with Kymriah + alloHCT was $1,263,316 ($696,768-2,404,447).
Factors associated with higher inpatient cost were public insurance ($96,287, p=0.002); inpatient Kymriah infusion ($395,182, p<0.001); CRS grade≥3 ($342,176, p<0.001); ICANS grade ≥3 ($354,013, p=0.010); ICU stay ($346,737 p<0.001). Multivariable analysis demonstrated that only inpatient infusion of Kymriah significantly associated with higher cost (Table 2).
Conclusion: In this cost analysis across CAYA receiving Kymriah, we describe the total inpatient treatment-associated cost, with inpatient infusion emerging as a main driver of cost. With increased clinical experience, outpatient infusions and early interventions to avoid high-grade toxicity and ICU-level care have become more common and potentially more cost-effective. Our data justifies efforts to deliver Kymriah in the outpatient setting, when safe and feasible, and to provide support systems to facilitate this. Additionally, Kymriah drug cost accounts for the bulk (73%) of the overall median cost, highlighting that efforts to lower drug costs may permit enhanced access globally.
Disclosures
Satwani:Sobi: Consultancy; Mesoblast: Consultancy. Phelan:Amgen: Research Funding; bluebird bio: Consultancy. Laetsch:AITherapeutics: Consultancy; Bayer: Consultancy; Advanced Microbubbles: Consultancy; Massive Bio: Consultancy; Jazz Pharmaceuticals: Consultancy; GentiBio: Consultancy; Menarini: Consultancy; Novartis: Consultancy; Pyramid Biosciences: Consultancy; Treeline Bio: Consultancy. Verneris:Medexus: Membership on an entity's Board of Directors or advisory committees; Jazz: Membership on an entity's Board of Directors or advisory committees; Qihan: Membership on an entity's Board of Directors or advisory committees; Sanofi: Membership on an entity's Board of Directors or advisory committees; Allovir: Consultancy; Forge: Consultancy; Omeros: Consultancy; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees. Fabrizio:Adaptimmune: Consultancy. Baumeister:Takeda: Other: Husband is an employee at Takeda. Qayed:Novartis: Honoraria; Vertex: Honoraria. Mackall:Link Cell Therapies: Consultancy, Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees; Lyell Immunopharma: Current equity holder in private company, Research Funding; Adaptimmune: Consultancy; Immatics: Consultancy; CARGO: Consultancy, Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees; Mammoth: Consultancy, Current equity holder in private company. Schultz:Novartis: Consultancy; cargo: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal